- Prospective Students
- About the GSBS
- MS PROGRAMS
- PHD PROGRAMS
- MD/PhD PROGRAM
- MD/PhD Program
- Participating Institutions/Entities
-
Student Research Day 2020
- Admissions
- Admissions
- Admission FAQs
- What factors are considered in admissions decisions?
- What is the minimum GPA required to apply?
- Do you require interviews?
- When will I be notified regarding interviews?
- What are the application deadlines?
- How long are my TOEFL scores valid?
- 我怎样才能得到一个助教奖学金?
- How can I get an assistantship if I am seeking a MS degree?
-
Admissions Office
6767 Bertner Avenue
S3.8344 Mitchell BSRB
Houston TX 77030
- beplay苹果手机能用吗
- beplay苹果手机能用吗
- Research Interests
-
Student Research Day 2020
- Student Life
- Current Students
- Faculty
- Alumni
- Academics
- Diversity
- Give
- Events
- Map
- Contact Us
- About the GSBS
About the GSBS
Quick Facts
- MS PROGRAMS
Individualized MS Program in Biomedical Sciences
- PHD PROGRAMS
PhD Programs
- MD/PhD PROGRAM
MD/PhD Program
Participating Institutions/Entities
- Admissions
Admissions
Admission FAQs
- What factors are considered in admissions decisions?
- What is the minimum GPA required to apply?
- Do you require interviews?
- When will I be notified regarding interviews?
- What are the application deadlines?
- How long are my TOEFL scores valid?
- 我怎样才能得到一个助教奖学金?
- How can I get an assistantship if I am seeking a MS degree?
Admissions Office
6767 Bertner Avenue
S3.8344 Mitchell BSRB
Houston TX 77030 - beplay苹果手机能用吗
beplay苹果手机能用吗
Research Interests
Student Research Day 2020
- Student Life
Student Life
Student Organizations
Faculty:
Grant Resources
GSBS provides this information to assist you during the grant application process. For more specific information regarding our parent institutions, please visit the "Related Links" section at the bottom of the page.
PA-21-071provides Research Supplements to Promote Diversity in Health-Related Research (Administrative Supplement, Clinical Trial Not Allowed) for a variety of research, program, and cooperative grants. The GSBS Deans can provide the "Applicant Eligibility Statement", verifying the GSBS student meets the eligibility criteria.Please allow one week for us to generate the letter.This letter will contain signature lines for the grant PI and the Institutional Signing Official (at UT MDACC OSP or UTHealth SPA). Submission dates vary by Institute/Center.
To request a letter, please send the following toNicolle Patterson.
1. the grant number, Institute/Center, and PI name
2. the student's full name and history of prior/current PHS support [if any, include grant numbers and dates of support]
NIH progress reports must describe the use of Individual Development Plans (IDPs). Per NIHNOT-OD-14-113:
NIH progress reports using the Research Performance Progress Report (RPPR) must include a report on the use of IDPs in Section B. Accomplishments, Question B.4. Actual IDPs should not be included. Instead, grantees will report on whether they use IDPs for all the graduate students and postdoctoral researchers included in Section D. List of Participants. The use of IDPs as well as the manner in which IDPs are used is expected to be determined by the awardee institution, but the RPPR will include a brief description of how and whether IDPs are used to help manage the career development of students and postdocs associated with that award. A similar response is required for all T, F, K, R25, R13, D43, and other awards or award components designed to provide training and professional development opportunities for graduate students and postdoctoral researchers.
A description of GSBS initiatives that meet this IDP/Career Development requirement can be foundhere.
Per NIHNOT-OD-16-080, the newest NIH Biosketch format is required for all grant submissions on or afterMay 25, 2016. NIH Biosketch templates, instructions and examples can be foundhere. The Science Experts Network Curriculum Vitae (SciENcv) has been modified to support the new biosketch format. SciENcv instructions can be found in thisYouTube video.
Find Funding Opportunities:We regularly monitor announcements of institutional training grant funding opportunities. We can help you find a funding source for your program. NIH is currently offering a number of T32s (see alsoNIH T Kiosk):
PA-20-142NRSA Institutional Research Training Grant [Parent T32]
Submission Dates: January 25, May 25, September 25 (annually)
PAR-17-341NIGMS NRSA Predoctoral Institutional Training Grant (T32)
Submission Dates: January 25, May 25, September 25 (annually)
PAR-18-524Institutional Training Programs to Advance Translational Research on Alzheimer's Disease and AD Related Dementias (T32)
Submission Dates: January 25, May 25, September 25 (annually)
RFA-HL-19-023T32 Training Program for Institutions That Promote Diversity
Submission Dates:9月4日2020; February 26, 2021
PAR-19-211NINDS Institutional Research Training Program (T32)
Submission Dates: May 27, 2020; May 26, 2021
PAR-19-228Institutional Translational Research Training Program (T32)
Submission Dates:May 27, 2020; May 26, 2021
PAR-20-076Jointly Sponsored Ruth L. Kirschstein National Research Service Award Institutional Predoctoral Training Program in the Neurosciences (T32)
Submission Dates:May 26, 2020; May 25, 2021; May 25, 2022
Application Modules:We have developed various modules for standard application sections. Modules include descriptions of GSBS initiatives coupled with NIH T32 application requirements. However, we are able to provide these modules for applications to any agency. GSBS initiatives should be integrated into your program-specific curriculum. Modules include:
-Degree Requirements
-Career Development
-Diversity Recruitment & Retention
-Responsible Conduct of Research
-Training Budget (& mock justification)
Data Tables:A training grant application requires extensive data reporting. GSBS maintains student and faculty databases and can provide most of the required data in the agency-specified format.
Application Library & Best Practices:We have been amassing a library of recently-submitted applications with corresponding reviewer comments, which allows us to establish, update, and share thematic best practices for training grant applications.
Letter of Support:’院长欣赏所需的奉献of Faculty to propose and maintain training programs for the benefit of GSBS students.
Let’s get started!!
Here is a sample timeline for a standard NIH T32 submission:
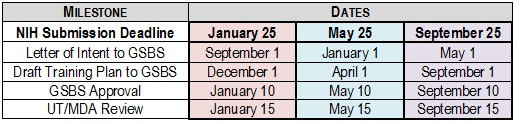
Letter of Intent:Please submit a Letter of Intent to the GSBS usingthis LOI format least 4 monthsprior to the agency deadline. We will provide initial feedback on your training plan and begin data table completion based on this information.
Draft Training Plan:Please submit a draft of your training plan for feedbackat least 8 weeksprior to the agency deadline.
GSBS Review and Approval:Please submit a final copy of your training plan and data tables for approval5 daysbefore institutional deadline.
Contact:Please direct correspondence toNicolle Patterson.
- NIH Supplements
PA-21-071provides Research Supplements to Promote Diversity in Health-Related Research (Administrative Supplement, Clinical Trial Not Allowed) for a variety of research, program, and cooperative grants. The GSBS Deans can provide the "Applicant Eligibility Statement", verifying the GSBS student meets the eligibility criteria.Please allow one week for us to generate the letter.This letter will contain signature lines for the grant PI and the Institutional Signing Official (at UT MDACC OSP or UTHealth SPA). Submission dates vary by Institute/Center.
To request a letter, please send the following toNicolle Patterson.
1. the grant number, Institute/Center, and PI name
2. the student's full name and history of prior/current PHS support [if any, include grant numbers and dates of support] - NIH Progress Reports
NIH progress reports must describe the use of Individual Development Plans (IDPs). Per NIHNOT-OD-14-113:
NIH progress reports using the Research Performance Progress Report (RPPR) must include a report on the use of IDPs in Section B. Accomplishments, Question B.4. Actual IDPs should not be included. Instead, grantees will report on whether they use IDPs for all the graduate students and postdoctoral researchers included in Section D. List of Participants. The use of IDPs as well as the manner in which IDPs are used is expected to be determined by the awardee institution, but the RPPR will include a brief description of how and whether IDPs are used to help manage the career development of students and postdocs associated with that award. A similar response is required for all T, F, K, R25, R13, D43, and other awards or award components designed to provide training and professional development opportunities for graduate students and postdoctoral researchers.
A description of GSBS initiatives that meet this IDP/Career Development requirement can be foundhere.
- NIH Biosketch Format
Per NIHNOT-OD-16-080, the newest NIH Biosketch format is required for all grant submissions on or afterMay 25, 2016. NIH Biosketch templates, instructions and examples can be foundhere. The Science Experts Network Curriculum Vitae (SciENcv) has been modified to support the new biosketch format. SciENcv instructions can be found in thisYouTube video.
- Training Grants
Find Funding Opportunities:We regularly monitor announcements of institutional training grant funding opportunities. We can help you find a funding source for your program. NIH is currently offering a number of T32s (see alsoNIH T Kiosk):
PA-20-142NRSA Institutional Research Training Grant [Parent T32]
Submission Dates: January 25, May 25, September 25 (annually)
PAR-17-341NIGMS NRSA Predoctoral Institutional Training Grant (T32)
Submission Dates: January 25, May 25, September 25 (annually)
PAR-18-524Institutional Training Programs to Advance Translational Research on Alzheimer's Disease and AD Related Dementias (T32)
Submission Dates: January 25, May 25, September 25 (annually)
RFA-HL-19-023T32 Training Program for Institutions That Promote Diversity
Submission Dates:9月4日2020; February 26, 2021
PAR-19-211NINDS Institutional Research Training Program (T32)
Submission Dates: May 27, 2020; May 26, 2021
PAR-19-228Institutional Translational Research Training Program (T32)
Submission Dates:May 27, 2020; May 26, 2021
PAR-20-076Jointly Sponsored Ruth L. Kirschstein National Research Service Award Institutional Predoctoral Training Program in the Neurosciences (T32)
Submission Dates:May 26, 2020; May 25, 2021; May 25, 2022
Application Modules:We have developed various modules for standard application sections. Modules include descriptions of GSBS initiatives coupled with NIH T32 application requirements. However, we are able to provide these modules for applications to any agency. GSBS initiatives should be integrated into your program-specific curriculum. Modules include:
-Degree Requirements
-Career Development
-Diversity Recruitment & Retention
-Responsible Conduct of Research
-Training Budget (& mock justification)Data Tables:A training grant application requires extensive data reporting. GSBS maintains student and faculty databases and can provide most of the required data in the agency-specified format.
Application Library & Best Practices:We have been amassing a library of recently-submitted applications with corresponding reviewer comments, which allows us to establish, update, and share thematic best practices for training grant applications.
Letter of Support:’院长欣赏所需的奉献of Faculty to propose and maintain training programs for the benefit of GSBS students.
Let’s get started!!
Here is a sample timeline for a standard NIH T32 submission:

Letter of Intent:Please submit a Letter of Intent to the GSBS usingthis LOI format least 4 monthsprior to the agency deadline. We will provide initial feedback on your training plan and begin data table completion based on this information.
Draft Training Plan:Please submit a draft of your training plan for feedbackat least 8 weeksprior to the agency deadline.
GSBS Review and Approval:Please submit a final copy of your training plan and data tables for approval5 daysbefore institutional deadline.
Contact:Please direct correspondence toNicolle Patterson.
Questions?
For Faculty-related information, please contactGSBS Faculty Affairs









